The second ATMP Supply Chain Conference, organized by at.las on February 8, brought together more than 120 specialists from both the logistics sector and the pharmaceutical industry. The event featured engaging discussions and insightful presentations focused on the manufacturing and transportation of Advanced Therapy Medicinal Products (ATMPs), personalized medicines that hold significant promise in modern medicine.
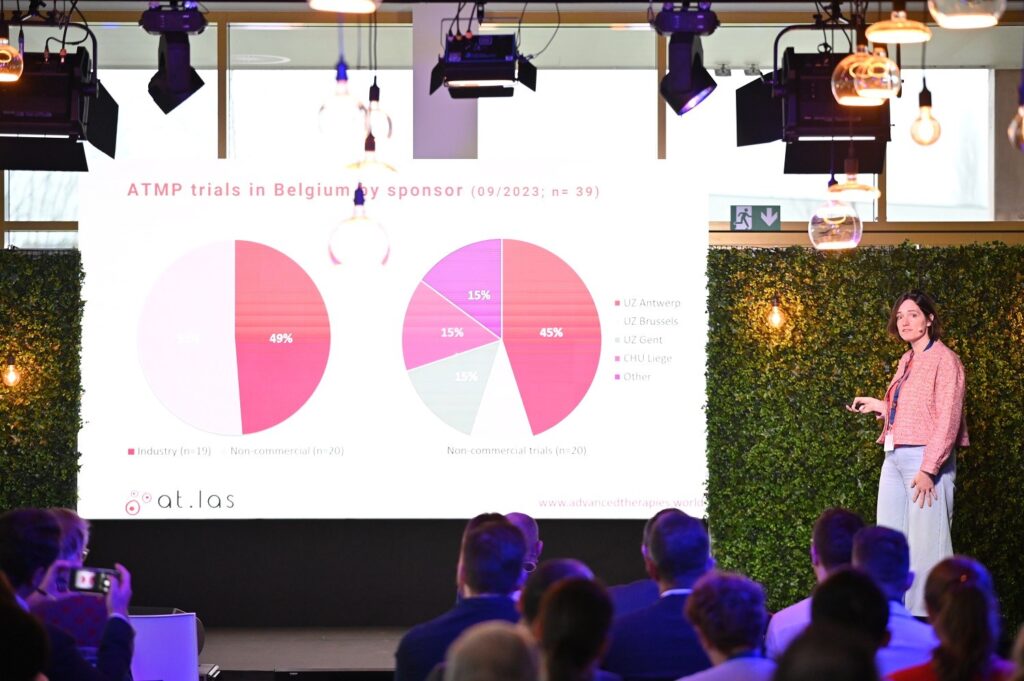
Keynote speaker Prof. Dr. Nathalie Cools, the founder of anicells, kicked off the day with an overview of the ATMP landscape in Flanders, providing valuable insights into the current state and future prospects of the industry. Following this, expert panels delved into the various challenges associated with manufacturing and transporting personalized medicines, fostering discussions aimed at identifying solutions and best practices.
During the afternoon session, representatives from Galapagos, Janssen Pharmaceutics, Pharma.aero (a collaborative platform for pharmaceutical transport), and Deliver-IT shared their experiences in establishing robust and resilient logistics chains specifically tailored to the unique requirements of cell and gene therapy products.
In a parallel session, ATMP developers learned how to improve their production process using next-generation, closed and automated platforms. anicells presented the results of a European-funded project during which they installed focused multiple platforms into their existing GMP-certified cleanrooms, providing cell therapy developers support to integrate these new platforms into the development and manufacturing of their cell therapy products. Additionally, users of different platforms shared their firsthand experiences, outlining the benefits and optimal scenarios for deploying these advanced platforms in cell therapy manufacturing workflows.
Overall, the conference provided a platform for knowledge exchange, collaboration, and innovation within the ATMP supply chain ecosystem, driving advancements aimed at enhancing the accessibility, efficiency, and reliability of personalized medicine manufacturing and distribution.